13 Work How To Find Concentration In A Solution - Recall that, in general, concentration tells you how much solute is present in a solution. This worked example shows how to determine the concentration of individual ions in an aqueous solution from the total concentration.

How to find concentration in a solution
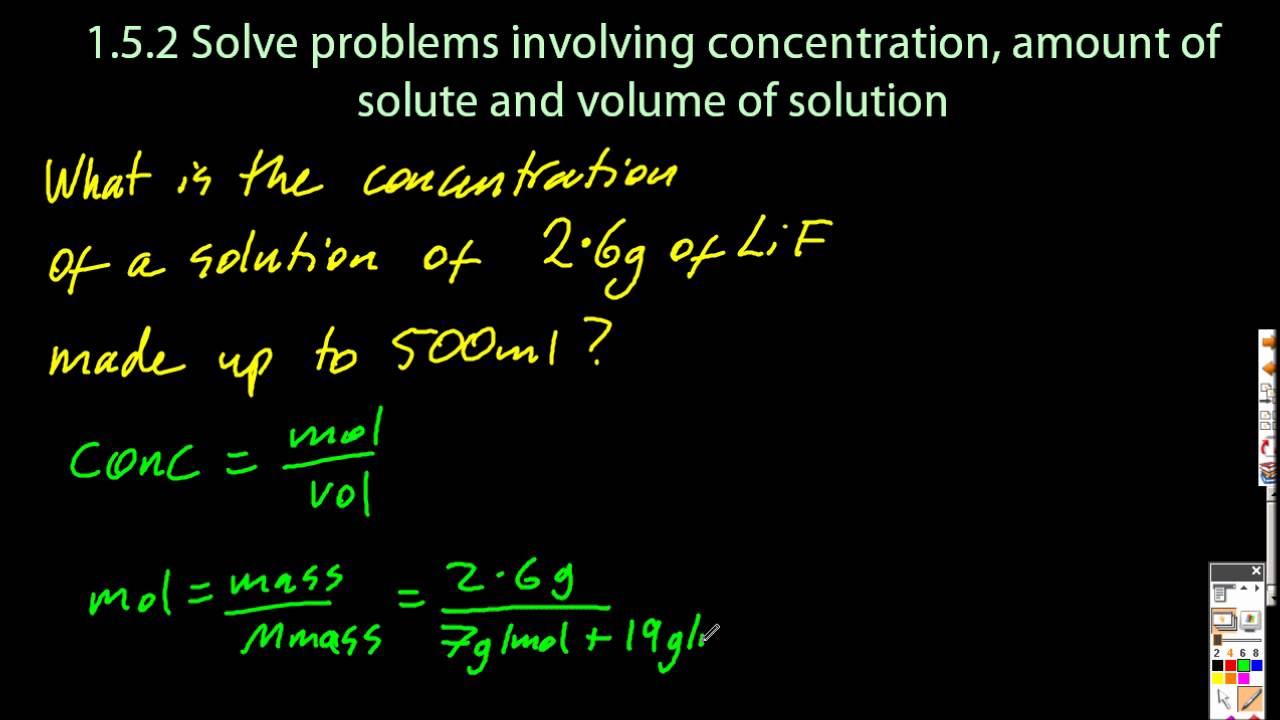
13 Absolutely How To Find Concentration In A Solution. It is denoted by x. A concentration in parts per million (ppm) tells you how many parts of solute are present in 1 000 000 parts of solution. So, c% = msolute msolution ⋅ 100%, where. How to find concentration in a solution
How to calculate molality of a solution molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Now that you know how to find the concentration of a solution using various concentration of solution formulas, we will try to solve some concentration of solution questions. How beer’s law determine concentration of a solution? How to find concentration in a solution
Solved problems question 1) 2ml of water is added to 4g of a powdered drug. In this solution, we see that the hydroxyl ion concentration is 10−14/10−4 = 10−10. Calculating mass concentration step 1: How to find concentration in a solution
Percent concentration by mass is defined as the mass of solute divided by the total mass of the solution and multiplied by 100%. First, divide the amount of the solute by the amount of the whole solution to get a “parts per 1” measurement for the. Calculating the concentration of solution1. How to find concentration in a solution
Suppose we have a solution containing as a You can use beer's law, which is a=e x. This is also referred to as molarity, which is the most common method of expressing the concentration of a solute in a solution. How to find concentration in a solution
The volume of this standard solution needed to do this is recorded. The ph of a solution is equal to the negative logarithm of the hydronium ion (h3o+) concentration. Identify the mass of the solute. How to find concentration in a solution
In this way, how do you find the concentration of an unknown solution? Msolution = msolvent + msolute. Divide the moles of solute by the volume of the solution in liters. How to find concentration in a solution
Of a solute in a solution. In biomedical sciences and is a science writer. Finding the mass of solutesample problem: How to find concentration in a solution
Now the moles of the ag+. Molar solution concentration equation.c is the molar concentration in mol/l (molar or m). The unit you use depends on the chemical solution. How to find concentration in a solution
The concentration of ions in solution depends on the mole ratio between the dissolved substance and the cations and anions it forms in solution. Volume% of a = v olume of component a in the solution t otal volume of the solution ×100 v o l u m e o f c o m p o n e n t a i n t h e s o l u t i o n t o t a l v o l u m e o f t h e s o l u t i o. Where the slope, m, is equal to εl. How to find concentration in a solution
The concentration can be expressed as a percent of one component in the solution by volume, it is then called as volume percentage and is given as: If the solution has a solvent and the solute, a mole fraction gives a concentration as the ratio of moles of one component to the total moles present in the solution. In this case, use the absorbance found for your unknown, along with the slope of your best fit line, to determine c, the concentration of the unknown solution. How to find concentration in a solution
Identify the volume of solution. Set up your equation so the molarity m = mol/v, where mol is the number of moles of the solute and v is the volume of the solvent. How many grams of copper are i. How to find concentration in a solution
For a neutral solution, [h+] is 10−7, or ph = 7. There are two ways to change a solution's concentration by mass. I think it is hard, maybe impossible, to calculate the concentration of your doped nanoparticles by mr. How to find concentration in a solution
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. A one peso coin has a mass of 5.4 grams. For larger hydrogen ion concentrations, then, the ph of the solution is < 7. How to find concentration in a solution
Divide the mass of the solute by the volume of solution to find the mass. Then you can use the following formula to find the concentration: So, if you have a compound that dissociates into cations and anions, the minimum concentration of each of those two products will be equal to the concentration of the original compound. How to find concentration in a solution
The equation for beer’s law is a straight line with the general form of y = mx +b. How to find concentration in a solution



![[Example] How to Find the Concentration of a Solution](https://64.media.tumblr.com/6baa6d4d4fcfae8bae629084f22c3996/760b3d8e38a5c8e7-57/s540x810/30e85284164ecadb9eafff749db3eb35e108cb3d.gifv)

![Given [H+] or [OH], Calculate pH & pOH YouTube](https://64.media.tumblr.com/6baa6d4d4fcfae8bae629084f22c3996/760b3d8e38a5c8e7-57/s540x810/30e85284164ecadb9eafff749db3eb35e108cb3d.gifv)



